Breakthrough HIV Prevention Injection Receives Approval
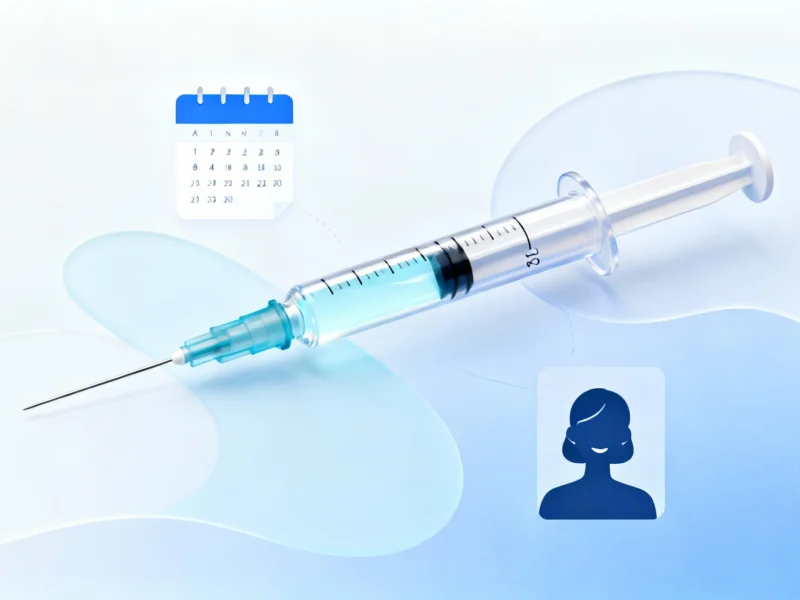
The United Kingdom has approved its first injectable medication for HIV prevention, according to health authorities, representing what Wes Streeting, the Secretary of State for Health and Social Care, described as a “game-changing” development in public health. The injection, which provides months of protection against HIV transmission, offers an alternative for people who cannot consistently use daily oral prevention pills.
Addressing Barriers to HIV Prevention
Traditional pre-exposure prophylaxis (PrEP) in pill form has been available for years and remains highly effective at preventing HIV/AIDS infections, but sources indicate significant barriers prevent some populations from consistent use. According to reports, challenges include difficulty accessing medications, practical obstacles, and concerns about privacy, with some individuals worrying that family members or housemates might discover their pills.
Analysts suggest that circumstances such as homelessness and domestic violence can make daily pill regimens particularly challenging to maintain. The new injection, which lasts for months between doses, reportedly offers both convenience and discretion that could benefit vulnerable populations who have struggled with oral PrEP adherence.
Government Commitment to Cutting-Edge Treatments
Health Secretary Wes Streeting stated that the approval “perfectly embodies what this government is determined to deliver – cutting-edge treatments that save lives and leave no one behind.” He emphasized that “for vulnerable people who are unable to take other methods of HIV prevention, this represents hope.”
The report states that the injection should be used in combination with safer sex practices, such as condom use, for comprehensive protection against HIV transmission. HIV remains a virus that damages immune system cells and weakens the body’s ability to fight infections and diseases, primarily transmitted through unprotected sex or shared needles.
Economic and Healthcare Implications
The NHS has reportedly secured an undisclosed discount from the manufacturer for the treatment, which carries a list price of approximately £7,000 per patient annually. This development comes amid broader global health and economic considerations, including ongoing international trade tensions that can affect pharmaceutical markets and technological advancements influencing healthcare innovation.
Meanwhile, early results for a different injection called lenacapavir suggest it may eventually enable annual HIV prevention dosing, according to research reports. This aligns with trends in healthcare sector developments and increasing integration of advanced technologies in medical research.
Expanding Prevention Options for At-Risk Populations
The availability of injectable HIV prevention represents a significant expansion of options for at-risk populations. Medical experts suggest that having multiple prevention modalities allows healthcare providers to tailor approaches to individual circumstances and needs, potentially increasing overall prevention effectiveness across diverse communities.
According to public health analysts, this approval marks an important step forward in the global effort to reduce HIV transmission rates, particularly among populations that have historically faced barriers to consistent prevention method use. The development reflects ongoing evolution in approaches to managing and preventing serious health conditions through innovative pharmaceutical solutions.
This article aggregates information from publicly available sources. All trademarks and copyrights belong to their respective owners.